The new JN.1 variant of COVID-19 has been spreading rapidly around the world. Notably, mainland China has witnessed a surge in crowded children's hospitals, reporting cases of "white lung syndrome" or "white lung" pneumonia and increased deaths since November. Similar occurrences were noted in the United States. Are they purely coincidental or is there a link between these two events?
Quickly Becoming a Global Trend
According to the Centers for Disease Control and Prevention (CDC), in the United States, JN.1 rose from less than 0.1 percent of SARS-CoV-2 circulating viruses at the end of October to 15–29 percent as of Dec. 8.
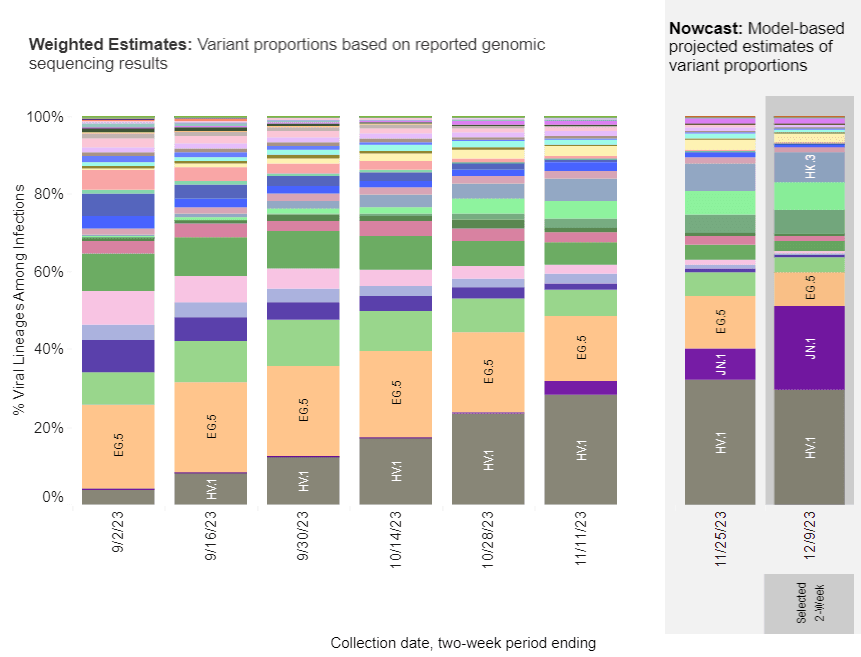
It also spreads in Europe, including the United Kingdom, France, and Asia.
JN.1 Has Tricks
What's particularly concerning about JN.1 is its notable divergence from other recently prevalent variants.
Compared to XBB.1.5, JN.1 contains 41 additional unique mutations, with most of the changes, i.e., 28 located in the spike protein, one in the N protein, three in the M protein, eight in the ORF1a, and one in ORF 7b.
Dr. Ishii and other Japanese researchers used a kind of virus-like particle to mimic the infection process of a virus, with results showing that JN.1 has a significantly increased infectivity compared to Pirola, according to the preprint paper published in December.
Weakened Protection by XBB.1.5 Vaccine
In simpler terms, the virus adapts, making it harder for our immune system to fight it off, even if people have been vaccinated or had previous COVID-19 infections.
Notably, JN.1 contains a new mutation of L455S, making the latest XBB.1.5 vaccine less likely to protect against JN.1.
A study by Dr. Ho from Columbia University revealed that individuals not previously infected who received a single mRNA vaccine based on XBB.1.5 showed a twenty-sevenfold increase in neutralizing antibodies. However, it is also worth noting that the binding strength of antibodies generated by subjects against those new variants emerged after XBB.1.5 weakened, especially for the JN.1 variant, where the neutralizing effects were one of the lowest.
Additional studies from Dr. Cao in those with breakthrough infections after XBB.1.5 vaccination confirmed again that there are relatively low antibodies generated against JN.1. Dr. Ishii and Dr. Cao have discussed how the new mutation of L455S on JN.1 significantly increases antibody evasion.
Increased Immune Escape Potential
Each cell has its method of efficiently recognizing and eliminating the virus. This recognition process is based on unique virus parts identified by each cell type.
To picture this, think of your hand with five fingers. When you wear gloves, each finger goes into a specific part of the glove. Similarly, when a virus enters our body, each immune cell identifies a distinct part of its surface and attacks it differently.
If a virus' spike protein mutates, the antibodies produced by significant immune cells—B-cells may not be able to fight against the mutated virus effectively. On the other hand, if a virus mutates in the non-spike protein, another primary type of immune cell—T-cells, may be unable to protect against the variant.
In the case of JN.1, changes in the spike protein contribute to its increased infectivity and escape from vaccine or infection-induced neutralizing antibodies.
The non-spike protein mutations are also alarming. For example, mutations in nonstructural proteins responsible for the RNA process have essential implications for the virus's life cycle. They can lead the virus to escape more quickly from a broader spectrum of immunity and other antiviral defense mechanisms that clear it from our cells.
In summary, mutations in the more viral proteins could enhance the virus's ability to escape from established immunity and cause disease, potentially making infections more severe.
As early as when Pirola spread in August, there was a concern that the variant with 34 mutations relative to BA.2 would "escape" T-cell immunity.
Italian immunologist Alessandro Sette at La Jolla Institute for Immunology at the University of California–San Diego found that with the immune response to the variant Pirola, two central types of T-cells (CD4 and CD8) are affected by 11 to 28 percent. For the spike protein, up to half were affected.
Although the authors remain optimistic about the potential protection of the XBB vaccine against Pirola, the virus has already learned to escape from the vaccine-induced or previous infection-induced T-cell immunity on top of B-cell immunity.
When additional JN.1 mutations continue to develop, knowing how much of the original T-cell immunity will be maintained to fight against the JN.1 remains an important question that warrants further investigation.
White Lung Syndrome in China and the US
Along with the rise of JN.1, a mysterious disease has also appeared.
Since late November, a surge of thousands of mysterious pneumonia cases has flooded into the hospitals of Beijing, Tianjin, and Shanghai. The large number of patients and severity of cases have raised an alarm similar to China's initial January 2020 COVID-19 outbreak.
The World Health Organization urged China for details of pathogens in this recent respiratory outbreak, according to Reuters; however, China initially claimed it was mainly caused by mycoplasma, which usually links to mild lung disease and is not categorized by China's Health Authority as an emerging infectious disease.
The symptoms of "white lung syndrome" (a folk name for severe pneumonia) include high fevers and unusual symptoms of silent hypoxia that are similar to COVID-19-associated pneumonia.
Reported cases in children with "white lung pneumonia" or "white lung syndrome" in the United States and Europe are escalating, increasing public concern alongside the rising JN.1 curve.
Coincidentally, Ohio’s Warren County experienced an “extremely high number of pediatric pneumonia cases” with 142 cases of pediatric pneumonia since August, issuing an official alert on Nov. 29. On Dec. 5, a Detroit mom shared that her son appeared to have "white lung" pneumonia when "one of his lungs was completely white."
The onset of this mysterious pneumonia in August occurred in the same month that JN.1 was detected. Nothing is coincidental.
Although a medical analysis of the pathogen of this pneumonia is lacking, there are several red flags when we attribute these lung problems to mycoplasma.
The SARS-CoV-2 virus and mycoplasma are completely different pathogens, yet the symptoms of pneumonia caused by both appear to be similar—they attack different parts of the lung with different clinical courses.
The human lungs exhibit a structure extending from the larger trachea to smaller units like alveoli, supported by the interstitium between these alveoli.
SARS-CoV-2 gains entry through ACE2 receptors, which are distributed abundantly in alveolar cells, leading to pneumonia and marked by damage to both alveoli and the interstitium. Severe cases are evident as "large white lungs" on X-rays, signifying widespread inflammation.
In contrast, mycoplasma affects the bronchi, causing milder inflammation, usually without severe pneumonia. It is generally mild and improves in about one week.
Media reports on severe white lung cases resembling prolonged COVID-19 progression raise concerns.
On Dec. 15, the China CDC reported seven cases of JN.1 and did not rule out the potential for it to become the predominant strain in China. However, JN.1 was reported by Shanghai as early as Oct. 11.
China does not routinely test for the SARS-CoV-2 virus in patients with pneumonia; the actual number of JN.1 strains in China is unknown.
Many countries have relaxed their COVID-19 RNA testing protocols, particularly in cases involving children. The challenge arises when individuals, especially those with pneumonia symptoms, may not immediately consider undergoing a COVID-19 test. Without conducting a COVID-19 test, it becomes less likely to determine whether the JN.1 variant causes the infection.
This situation is prone to underestimating the prevalence of JN.1 cases outside of China. Moreover, within China, the problem of concealed cases and non-transparent reporting significantly skews the count of potential JN.1 cases. These issues contribute to a systemic underestimation of JN.1 cases within China.
Even though we do not have adequate case data to help us make better judgments about the link between these two “coincidental” phenomena this winter, the overlapping timing with a more complex mutation, more aggressive immune escape, and the potential increase in severity may all contribute to the possible link between them. This warrants a deeper medical and laboratory investigation of the current pneumonia cases, including SARS-CoV-2 gene sequencing.
The predominant lung issues are likely attributed to the current SARS-CoV-2 variants, potentially JN.1. Concurrent infections with other bacteria, including mycoplasma or viruses, are plausible, adding complexity to the wave of cases.
Future Reflections
A recent Austrian study covering over 3 million people shows that a fourth dose of the COVID-19 vaccine can increase the risk of COVID-19 death by 24 percent compared to those with three doses.
Government officials have yet to find effective measures to control the remaining COVID-19 surges.
The virus has proven resilient, mutating to counteract our vaccines. The critical question remains: How are we going to bring an end to this ongoing pandemic? Without understanding how to eradicate the virus at its core, it will merely persist in playing a cat-and-mouse game with humanity.
To address this unprecedented challenge, it's essential to revisit the question of the SARS-CoV-2 origin. Only by identifying the root cause can we effectively and swiftly resolve this issue.
Individually, we must focus on a healthy lifestyle to bolster our precious immunity in the future. Prioritizing healthy habits such as maintaining sufficient vitamin D levels, connecting with nature, cultivating inner peace, and showing consideration for others will strengthen our antiviral immunity.


No comments:
Post a Comment