A new research letter sponsored by Pfizer linked COVID-19 vaccination with reduced medical visits for respiratory illness in children. The detailed data, however, triggered doubt.
When compared to unvaccinated children, children younger than 5 years who received “at least 2 doses” of the original Pfizer vaccine were associated with "a reduced risk of COVID-19 emergency department or urgent care and outpatient visits,” the authors wrote.
Additional Dose Increased Risk
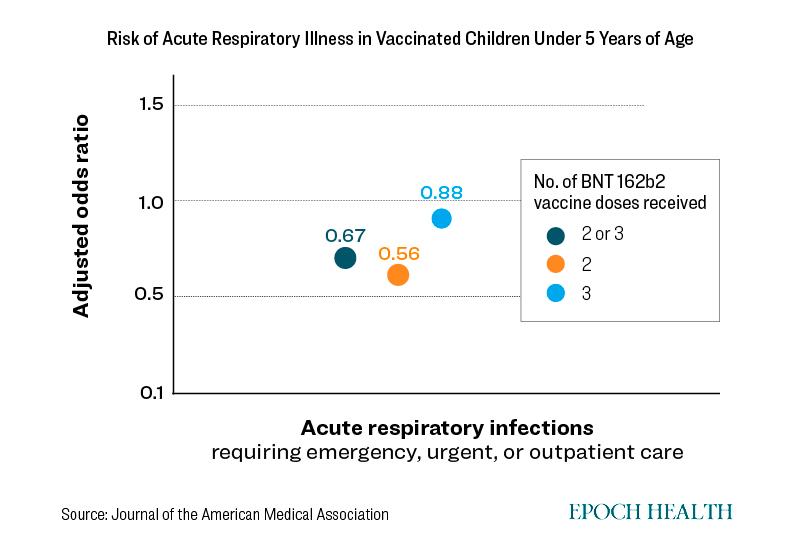
Children who were given two doses had a 46 percent lower risk of respiratory infection with COVID compared to unvaccinated children. However, children who were given three doses only had a 12 percent lower risk than unvaccinated children.This means these children had an increased risk of 34 percent compared to children who had two doses.
Furthermore, they had a confidence interval of 0.62 to 1.25 for risk of ambulatory visits.
A confidence interval below one indicates a reduced risk of ambulatory visits, while a confidence interval above 1 could indicate no benefit or possibly even increased risk of ambulatory visits.
"The 12 percent reduction is the best single estimate but what the confidence interval is telling you though, is that the study might have been underpowered to produce that effect," Dr. Andrew Bostom, retired associate professor of medicine at Brown University, told The Epoch Times.
Hence, the risk reduced is not "statistically significant," he added.
Dr. Bostom said that it is particularly important to drive home the message that children who took the three doses of the Pfizer vaccine had statistically insignificant benefits, since Pfizer and the Centers for Disease Control or Prevention (CDC) consider three doses rather two as the standard regimen for a child.
Children who receive Moderna vaccines require two doses to be considered fully vaccinated, whereas those who followed Pfizer's regimen need three doses.
Omicron: Less Lethal Than Previous Variants
Despite the authors' reasoning, studies have shown that Omicron tends to be less lethal than earlier variants.While the Wuhan, Alpha, and Delta variants infected the lower lung, inducing pneumonia and multisystemic inflammatory diseases that were much more severe in their symptoms and damage, Omicron was more inclined to infect the upper respiratory tract.
The diagnosis of acute respiratory illness referred to in the study can occur in the upper or lower respiratory tract. The authors did not say if they looked for lower or upper respiratory tract infections.
The earlier variants had a stronger dependency on an enzyme called TMPRSS2, which is more prevalently expressed in the lower lungs, which is why they tend to cause more lung damage.
Conflicts of Interest
Seven researchers and clinicians at Kaiser Permanente Southern California (KPSC) conducted the study, and six out of the seven had conflicts of interest with Pfizer.Four out of the seven authors were stockholders in Pfizer during the study and the other two received grants from Pfizer.
New Boosters Approved, No Data in Children
The new paper, published on Sept. 15, came days after the Food and Drug Administration and the CDC cleared and recommended new COVID-19 boosters for all Americans aged 6 months or older.The new Moderna vaccine was tested in 50 people with one person suffering an adverse event.
None of the vaccines were tested in children under 5.
Still, some advisers said it was safe to assume the vaccines will work in children.
"We don't have a lot of data in children, but ... it's likely there will be a benefit," adviser Dr. Oliver Brooks said.




No comments:
Post a Comment